Sathish Srinivasan, Ph.D.
Professor
Cardiovascular Biology Research Program
My 101
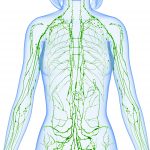
My lab is interested in understanding the formation and functioning of lymphatic vessels and valves.
The lymphatic vessels collect tissue fluids and digested lipids and deliver them to blood. Additionally, lymphatic vessels function as a conduit for inflammatory cells. Mutations in genes, surgery, radiation therapy or infections could damage lymphatic vessels. Defects in lymphatic vessels cause lymphedema, a disease characterized by the swelling of hands and legs. The health consequences of lymphedema include pain, inflammation, fibrosis, heart attack, obesity, atherosclerosis, high blood pressure, angiosarcoma and even aging and Alzheimer’s disease. Millions of people suffer with lymphedema. However, there are currently no treatments for this debilitating disease.
Lymphatic vessels, veins and heart contain valves to regulate the unidirectional flow of blood and lymph. Defects in valves lead to a wide range of diseases such as lymphedema, chylous ascites, spider veins, varicose veins, deep vein thrombosis, calcific aortic valve disease and mitral valve prolapse. Approximately 2.5% of the entire population is afflicted with heart valve disease. The prevalence increases to 10% in people who are older than 65. The prevalence of venous valve disease is difficult to quantify due to broad variability in the disease spectrum. However, approximately 15% of men and 25% of women who are older than 15 years of age are estimated to have visible varicose veins.
My lab has determined that the lymphatic vessels and valves (lymphatic, venous and heart valves) have numerous similarities at the molecular level. Building on these findings we investigate the mechanisms of their development and disease. Specifically, the major goals of my lab are to: A) Develop approaches to gain a clearer understanding of lymphatic vessel and valve development. B) Generate mouse models of human disease and identify the anatomical, developmental and functional defects in these mice. C) Develop innovative treatments for cardiovascular disorders.
Research
My goals are to understand the molecular mechanisms regulating lymphatic and valvular endothelial cell development and maintenance.
The mammalian lymphatic vasculature is important for returning the extravasated plasma fluids in tissue space back to blood circulation, absorption of digested lipids from the intestine and in immune surveillance. Lymphedema is a disfiguring and mobility restricting disease that results due to the abnormal functioning of the lymphatic vasculature. Lymphedema is a common disorder that results either due to mutations in genes that regulate lymphatic vascular development or more often due to infection or surgical damage to the lymphatic vessels. Lymphatic vasculature is also considered as a major route for tumor metastasis especially melanoma and breast cancer. Despite its importance, diagnosis of lymphatic disorders and the identification of new targets to treat those ailments are limited.
Valves regulate the unidirectional flow of fluids (blood or lymph) in the mammalian vascular system. Valves are present in the heart, veins and the lymphatic vessels and are crucial for the normal physiology of the cardiovascular system. In addition, there is also a unique type of valve known as the lymphovenous valve that regulates the return of lymph fluid including all of the digested lipids (approximately 2 liters per day in an average human being) back to venous circulation.
Congenital cardiac valve defects are common causes for childhood mortality and morbidity. Calcification of cardiac valves occurs in approximately 10% of people who are at least 65 years old. This leads to valvular stenosis that could result in atherosclerosis, pulmonary edema, thromboembolism, cardiac hypertrophy and sudden cardiac death. A high-fat diet is known to aggravate this disease. Infection and non-infection (cancer) related endocarditis also damages cardiac valves.
In the aging population a range of venous valve defects such as spider veins, varicose veins and venous insufficiency are common. Incompetent venous valves results in ineffective blood flow causing pooling of blood and thrombosis. This result in embolism, problems in wound healing and tissue necrosis. In severe cases this leads to the stroke or amputation of the limbs.
Defects in formation or functioning of lymphatic valves cause lymphedema. Lymphectomy performed during breast cancer treatment frequently damages both lymphatic vessels and valves resulting in lymphedema.
No information is currently available regarding lymphovenous valves in human disease although using mouse models, we have recently demonstrated that defects in these valves could lead to lymphedema and chylothorax (Geng, et al 2016). We believe that similar to the other cardiovascular valves they are likely to undergo aging related degeneration.
Despite their importance our understanding of valves during normal development and in disease is limited. With increase in the median age of the general population and with improving treatment options for cancer we have an urgent need to better understand the biology of valves and develop treatment methodologies to treat valve disorders beyond conventional synthetic replacement valves that are associated with significant mortality and morbidity.
Despite their differences, the development of both lymphatic and valvular endothelial cells is regulated by a common set of transcription factors and signaling molecules. Our goal is to understand these molecular mechanisms and translate this knowledge into effective treatments.
Brief CV
Education
B.Tech., Anna University, Chennai, India, 1999
Ph.D., Tulane University Medical Center, New Orleans, LA, 2003
Honors and Awards
Morris F. Shaffer and Margret H.D. Smith-Shaffer Award for Excellence in Research, Tulane University, New Orleans, LA, 2003
Keystone Symposia Scholarship, Keystone Symposium on Molecular Targets for Cancer Therapy, Banff, Canada, 2003
Award for Best Scientific Talk, Vanderbilt University retreat on developmental biology, Paris Landing State Park, TN, 2005
Outstanding Poster Award, Developmental vascular biology workshop II, North American Vascular Biology Organization, Asilomar, CA, 2006
Travel Award, Developmental vascular biology workshop III, North American Vascular Biology Organization, Asilomar, CA, 2008
Travel Award, Developmental vascular biology workshop IV, North American Vascular Biology Organization, Asilomar, CA, 2010
Fred Jones Award for Scientific Achievement, OMRF, 2017
Tucker Collins Lecture, Harvard University, Boston, MA, 2020
Membership
Oklahoma Center for Adult Stem Cell Research Steering Committee
North American Vascular Biology Organization
Society for Developmental Biology
American Heart Association
Joined OMRF scientific staff in 2013
Publications
Recent Publications
Kondo Y, Jiang Y, Geng X, Song J, Simeroth S, McDaniel JM, Yu P, Srinivasan RS, Xia L. Site-1 protease-mediated cholesterol metabolism is essential for lymphatic development in mice. JCI Insight 10, 2025 October, PMID: 41122969, PMCID: PMC12581667
Geng X, Chen L, Ahmed Z, Formigari GP, Ho YC, Del Gaudio I, Datilo MN, Azartash-Namin ZJ, Heron C, Shan X, Keshari RS, Pal S, Chen H, Lupu F, Xia L, Randolph GJ, Zawieja SD, Camerer E, Davis MJ, Srinivasan RS. S1PR1 regulates lymphatic valve development and tertiary lymphoid organ formation in the ileum. J Exp Med 222, 2025 September, PMID: 40553105, PMCID: PMC12187108
Davis MJ, Castorena-Gonzalez JA, Li M, Simon AM, Srinivasan RS. Hierarchical requirement for endothelial cell connexins Cx37, Cx47, Cx43 and Cx45 in lymphatic valve function. Function (Oxf), 2025 July, PMID: 40720769, PMCID: PMC12448487
Selected Publications
Chen D#, Geng X#, Lapinski PE, Davis MJ, Srinivasan RS*, King PD* (2020). RASA1-driven cellular export of collagen IV is required for the development of lymphovenous and venous valves in mice. Development. (cover image), Dec 7;147(23):dev192351. doi: 10.1242/dev.192351. PMID: 33144395, PMCID: PMC7746672. (# co-first authors; *Co-corresponding authors).
Cha B*, #, Ho YC#, Geng X, Mahamud MR, Chen L, Kim Y, Choi D, Kim TH, Randolph GJ, Cao X, Chen H, Srinivasan RS* (2020). YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling. Development. (cover image), Dec 13;147(23):dev195453. doi: 10.1242/dev.195453. PMID: 33060128, PMCID: PMC7758626. (# co-first authors; *Co-corresponding authors).
Geng X, Yanagida K, Akwii RG, Choi D, Chen L, Ho Y, Cha B, Mahamud MR, de Ruiz KB, Ichise H, Chen H, Wythe J, Hong YK, Mikelis CM, Hla T, Srinivasan RS (2020). S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling. JCI Insight. (cover image), Jul 23;5(14):e137652. doi: 10.1172/jci.insight.137652. PMID: 32544090, PMCID: PMC7453895.
Mahamud MR, Geng X, Ho YC, Cha B, Kim Y, Ma J, Chen L, Myers G, Camper S, Mustacich D, Witte M, Choi D, Hong YK, Chen H, Varshney G, Engel JD, Wang S, Kim TH, Lim KC, Srinivasan RS (2019). GATA2 controls lymphatic endothelial cell junctional integrity and lymphovenous valve morphogenesis through miR-126. Development. Nov 5;146(21):dev184218. doi: 10.1242/dev.184218. PMID: 31582413, PMCID: PMC6857586.
Cha B, Geng X, Mahamud MR, Zhang JY, Chen L, Kim W, Jho EH, Kim Y, Choi D, Dixon JB, Chen H, Hong YK, Olson L, Kim TH, Merrill BJ, Davis MJ, Srinivasan RS (2018). Complementary Wnt sources regulate lymphatic vascular development via PROX1-dependent Wnt/ß-catenin signaling. Cell Reports (Cover image). 25(3):571-584. PMID: 30332639, PMCID: PMC6264919.
Cha B, Geng X, Mahamud MR, Fu J, Mukherjee A, Kim Y, Jho EH, Kim TH, Kahn ML, Xia L, Dixon JB, Chen H, Srinivasan RS (2016). Mechanotransduction activates canonical Wnt/beta-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev. (cover image) 30(12):1454-69. PMID: 27313318, PMCID: PMC4926867.
Contact
Cardiovascular Biology Research Program, MS 45
Oklahoma Medical Research Foundation
825 N.E. 13th Street
Oklahoma City, OK 73104
Phone: (405) 271-3550
Fax: (405) 271-3137
E-mail: sathish-srinivasan@omrf.org
For media inquiries, please contact OMRF’s Office of Public Affairs at news@omrf.org.
Lab Staff
Karthik Hemantha Kumar, Ph.D.
Postdoctoral Scientist
Marcella Neves Datilo, Ph.D.
Postdoctoral Scientist
Guilherme Pedron Formigari, Ph.D.
Postdoctoral Scientist
Lijuan Chen
Manager Laboratory
Zheila Azartash-Namin
Research Technician III
Hayoung Cho
Research Technician II
Magdalena Karolak
Research Trainee
Frances Torres
Administrative Assistant I
News from the Srinivasan lab

June OMRF has welcomed military academy students to its labs since 2009. For Dr. John Saxon III, the connection was one worth making. “Some people may not think of basic science and the military as linked, but I thought that I could use OMRF’s work as an opportunity to stimulate some basic bench science interest with cadets […]