Can the coronavirus rewire the body’s immune system to trigger diseases like lupus?
By Adam Cohen | Illustration by Daniel Hertzberg | Lettering by Anna Heigh
When Covid-19 started its inexorable march around the globe in early 2020, Dr. Linda Thompson knew what she had to do.
An immunologist, Thompson had joined the Oklahoma Medical Research Foundation in 1989 from Scripps Research, where she’d been a faculty member. In the ensuing three decades, she’d investigated the immune system in a variety of ways. She’d studied a form of severe combined immunodeficiency known as “bubble boy” disease. She’d created laboratory mice that lacked an enzyme key to immune responses and shared them with scientific collaborators on four continents.
In a project for the National Institutes of Health, she’d examined how patients with lupus responded to flu shots. That led to a collaboration with other OMRF scientists to study another illness that, like lupus, causes the body to turn its immune system against itself.
As Covid-19 spread, Thompson set her sights on helping to fight the virus. “How could you not?” she says.
With cases mushrooming and time in short supply, scientists understood that developing the most effective strategies against a virus that hadn’t existed even a year ago meant building not from scratch but, rather, from existing research. For Thompson and her colleagues at OMRF, that pointed to a pair of beachheads.
“Because of the work we did in the flu vaccine, we had the infrastructure to measure the body’s immune response,” says Thompson, who holds the Putnam City Schools Distinguished Chair in Cancer Research at OMRF. “And because of our experience working with patients with lupus and other autoimmune diseases, it made sense for us to focus there.”
Teaming with Dr. Mark Coggeshall and other scientists at OMRF, she submitted a proposal to the NIH to expand upon immunology projects Coggeshall had led for many years. When OMRF received the funding, along with a gift from a group of local philanthropists intended to jump-start Covid-19 research in Oklahoma City, labs across OMRF kicked into action.
Overnight, immunologists, cell biologists and rheumatologists all became Covid researchers. Their projects varied, but as time passed, the most successful ones coalesced around a theme: the relationship between this novel virus and autoimmunity.
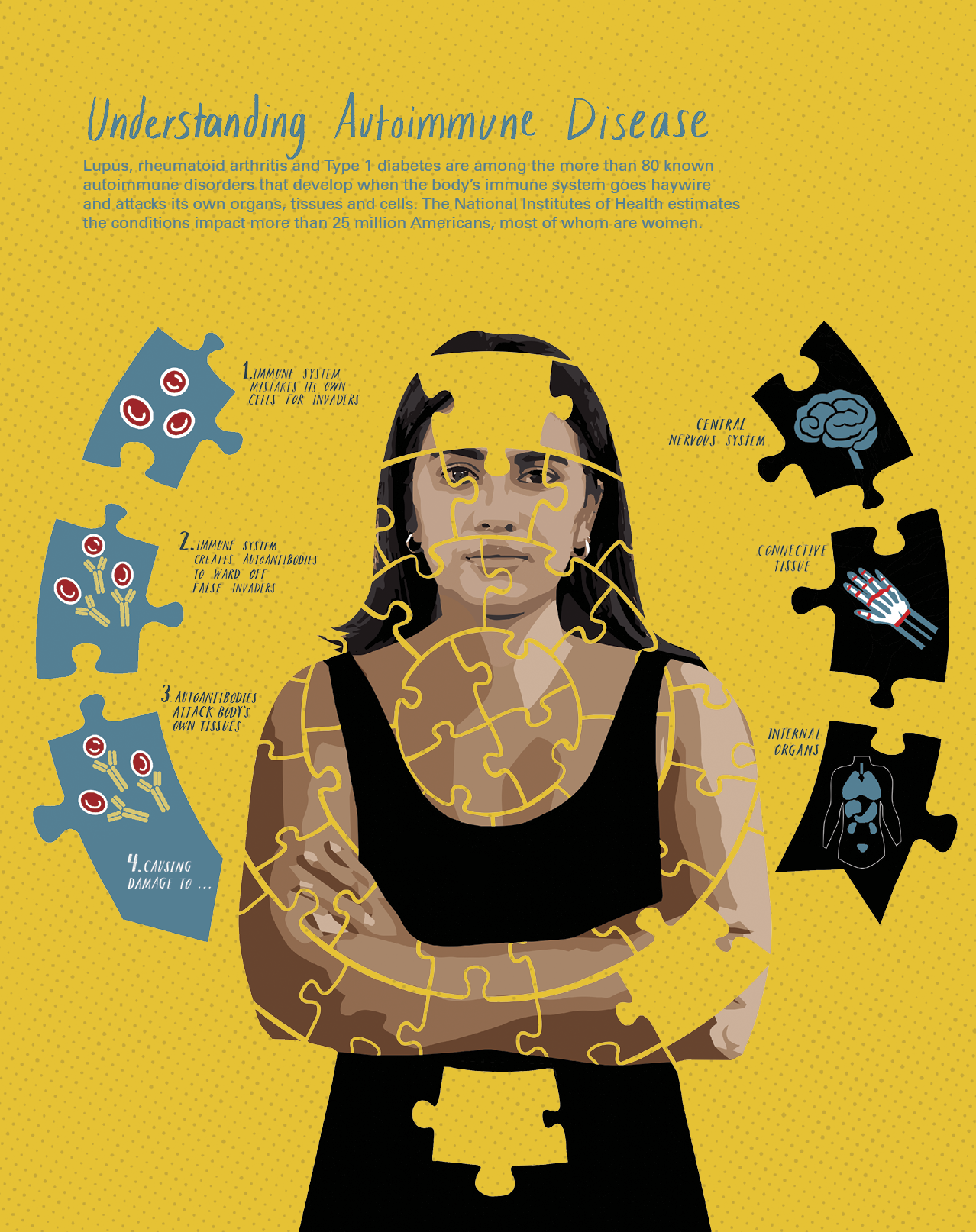
The phenomenon of autoimmunity occurs when the body confuses its own cells for invaders like viruses and bacteria. The immune system then unleashes powerful countermeasures against itself. Depending on the cells targeted, this can result in a variety of diseases, including lupus, multiple sclerosis and Type 1 diabetes. All told, these and roughly 75 other autoimmune illnesses affect an estimated 25 million Americans.
Like so many other conditions, the origins of these disorders are not precisely known. However, scientists have increasingly found evidence that viral infection can trigger their onset.
As Covid-19 cases swept across the country and world again and again, scientists at OMRF and elsewhere looked at the interplay between the virus and autoimmune diseases. In the course of their work, the researchers grappled with the obvious tolls of the virus: acute illness, death in some, lingering effects in others. But as their understanding has taken more precise shape, they’ve also begun to ask another question.
Can Covid-19 cause autoimmune disease?
_________

Twenty-five years ago, OMRF’s Dr. Judith James helped pioneer the idea that a virus might serve as the trigger for a chronic autoimmune disease. Trained both as a rheumatologist and an immunologist, James began her research career by looking at the causes of lupus, a disorder that’s notoriously difficult to diagnose due to the many different ways it presents in patients. Although the illness can cause the body’s immune system to lash out at numerous organs and systems, it most commonly strikes the joints and skin, and its dermatological symptoms most likely gave the illness its name: A 13th-century physician is reputed to have assigned the label “lupus” – Latin for wolf – to the condition because the facial lesions it caused reminded him of a wolf’s bite.
Beginning in the 1970s, scientists identified multiple genes that seemed to predispose people to the disease. However, they also suspected that some unknown environmental agent was operating as a sort of “on” switch for those genes, causing them to activate in certain people. In 1997, James, who is now OMRF’s vice president of clinical affairs and holds the foundation’s Lou C. Kerr Endowed Chair in Biomedical Research, led a study that identified a potential culprit.
Using blood samples donated by children and young adults, James and her then-OMRF colleague Dr. John Harley found that 99% of young patients with lupus had at some point in their lives been infected with Epstein-Barr virus, which is known to cause common colds in most and mononucleosis in some. But among healthy matched controls from the same age group, the researchers discovered that a much smaller percentage tested positive for the virus. When the scientists looked for a similar correlation with other cold viruses, they found none.
“These data are consistent with, but do not in themselves establish, Epstein-Barr virus infection as an etiologic” – causal – “factor in lupus,” James and Harley wrote when they published the results of their work in The Journal of Clinical Investigation, an influential scientific publication.
Even a quarter-century later, the study stands out among the hundreds and hundreds James has since done in her career. She recalls driving to a small city in southeastern Oklahoma to enroll a girl with pediatric lupus. When the girl’s softball teammates learned about the study, they all wanted to help their friend, so every one of them asked to enroll, too – as healthy controls. “Those are the kinds of papers,” says James, “you don’t forget.”
The work also proved unforgettable from a scientific perspective. “Judith James and John Harley did seminal work showing that Epstein-Barr virus plays a causative role in lupus,” says Dr. P.J. Utz, a professor of immunology and rheumatology at Stanford University. “That changed how we thought about autoimmunity in general.”
In the ensuing years, James continued to amass evidence that the virus represented a crucial piece in the puzzle that is lupus. She gathered new clues both in the clinic and at the cellular level, which pointed to links between Epstein-Barr infection and the subsequent onset of the autoimmune disease.
She hypothesized that in a certain subset of people, most likely due to their genetic profiles as well as other factors in their lives, “the body makes the wrong immune response to Epstein-Barr virus.” This confusion causes the immune system to create antibodies that target not only viral invaders but also the body’s own cells. In landmark research in The New England Journal of Medicine, James, Harley and OMRF colleague Dr. Hal Scofield demonstrated that these “autoantibodies” often appeared in the blood of seemingly healthy people who would later go on to develop lupus.
That landmark revelation, says Stanford’s Utz, made the study “one of the most impactful papers in autoimmunity.” The work offered the strongest proof yet that lupus and other autoimmune disorders don’t simply crop up overnight; those who develop the conditions often have preexisting autoantibodies that are like dominoes waiting to fall. “They’ve been percolating them for months or years,” Utz says.
As Covid-19 cases became widespread, James, Utz and other experts saw hints that infection with the novel coronavirus might be exactly the sort of nudge this group did not need. “With people who are predisposed to autoimmunity,” says Utz, “the virus might just push them over the edge.”
_________
When James and other researchers studied Covid-19 patients, they observed what she characterizes as “an overzealous immune response.” Much of the data, she says, “looked very similar to what we see in lupus.” Patients’ blood showed a low concentration of white blood cells, which is atypical of infections but consistent with lupus. On a molecular level, some of the same immune pathways that overreact in lupus and rheumatoid arthritis, another autoimmune disorder, were also overreacting with SARS-CoV-2 infection.
 Covid-19 can elicit a dizzying array of symptoms. But many – rashes, joint pain, severe fatigue, fever, swollen lymph nodes – mirror those found in lupus.
Covid-19 can elicit a dizzying array of symptoms. But many – rashes, joint pain, severe fatigue, fever, swollen lymph nodes – mirror those found in lupus.
Joining with Utz’s team at Stanford as well as researchers in Germany and the University of Pennsylvania, James dug deeper into the roots of those similarities. Specifically, the scientists examined the blood of patients who’d been hospitalized with Covid-19. They found that, compared with people who hadn’t been infected, the patients were much more likely to harbor autoantibodies.
Those autoantibodies were some of the same ones James had first demonstrated can serve as harbingers of autoimmune disease. So, says Utz, “If you get sick enough from Covid-19 to end up in the hospital, you may not be out of the woods even after you recover.”
The work offered a tantalizing clue that severe Covid-19 could ultimately trigger autoimmune disease. But it represented only a fraction of the work she’d launched into to explore the overlap between these two conditions.
As part of the effort to expand on the long-term immunology project led by OMRF’s Coggeshall, she looked at the immune responses of autoimmune disease patients who’d recovered from Covid-19. “We wanted to understand how individuals who did poorly after infection compared to those who did very well,” she says. She hoped she could “learn from the people who did well to see what part of the immune system needed to be shored up.”
In the clinic, she led an arm of an NIH trial testing various vaccination strategies in autoimmune patients. The trial focused on how to maximize the effect of boosters in people whose immune systems are compromised. “Should we switch to a different vaccine? And does it help to stop their medicines?”
In the course of her work, James expected to see that people with autoimmune disease had higher levels of severe Covid-19 than those without comorbidities. This didn’t turn out to be the case: Both groups showed similar levels of severe illness. However, in autoimmune patients, their symptoms lasted longer. And as reports emerged of long Covid – prolonged symptoms that linger or develop after the initial acute phase of infection – the syndrome once again echoed what she saw in her patients. “Fatigue, joint pain, rash. These are typical of autoimmune diseases we take care of as rheumatologists,” conditions such as fibromyalgia, lupus and rheumatoid arthritis.
She joined a national consortium led by New York University called RECOVER, which is studying multiple aspects of long Covid in patients over a four-year period. “Our goals are to identify treatments and other interventions to help patients who have long Covid symptoms. We also want to be able to predict people who are at the highest risk of developing long Covid,” she says.
With RECOVER, she’ll also stay alert for clues about how long Covid overlaps with autoimmunity. Already, a study from researchers at the Institute for Systems Biology has produced a pair of intriguing leads.
The work identified four factors that appear to place people at high risk for the syndrome. One is viral load, and another is having Type 2 diabetes. But it’s the last two that are of particular interest to James and her colleagues.
The scientists determined that patients were at greater risk for long-term symptoms if they had autoantibodies in their blood early in their coronavirus infection. Ditto if their bodies showed reactivation of a common pathogen: Epstein-Barr virus.
Autoantibodies, Epstein-Barr virus. As James has shown, both are also likely culprits, or at least accomplices, in autoimmune disease. Now, it’s up to her and other autoimmunity researchers to decipher their role in Covid-19. And then, of course, to use this information to blunt the impact of the virus and improve outcomes for patients worldwide.
“We are,” says James, “in a very challenging situation.”
_________
 James will continue to study Covid-19 through the lens of autoimmune disease. She’ll focus on how she can ensure the well-being of her patients with lupus and rheumatoid arthritis in the teeth of a viral pandemic that is becoming endemic.
James will continue to study Covid-19 through the lens of autoimmune disease. She’ll focus on how she can ensure the well-being of her patients with lupus and rheumatoid arthritis in the teeth of a viral pandemic that is becoming endemic.
But like others in her field, she also has her eye on the autoimmune implications of widespread coronavirus infection for the hundreds of millions of Americans who don’t have autoimmune conditions. Or at least not yet.
In the clinic, following coronavirus infections, she’s seen one patient develop lupus, another rheumatoid arthritis. As a scientist, she knows this is “clinical observation, not research.” Both might already have been on the road to autoimmune disease.
Still, that’s how research begins: with an observation. That observation becomes research when scientists put it through rigorous testing and analysis.
Moving forward, James is interested in finding people with autoantibodies – but not full-blown autoimmune disease – and following them clinically for the next few years. She wants to see what happens to those who get Covid-19 and those who don’t.
Will hospitalized patients, who disproportionately tested positive for autoantibodies in James’ study with Stanford’s Utz, go on to develop autoimmune disease at higher levels than the general population? How will the severity of a person’s illness factor into the equation? And what about long Covid, with the condition’s eerie resemblance to autoimmune disorders?
Finding answers to these and a host of other related questions will not be simple, says James. “We need hundreds of thousands of people who’ve been infected with the coronavirus and tens of thousands of people who have long Covid to understand whose autoantibodies go away, who progresses to autoimmune disease, and what the underlying mechanisms are.” There are, she says, “a lot of different clinical syndromes that get lumped together, so we have to have enough patients to tease out the many different pieces.”
While James searches, variants of Covid-19 will continue to run through the population. And with those infections, she predicts, will come more patients with joint problems, more with prolonged fatigue – “symptoms that look like what we see in autoimmune disease patients.”
Solving the puzzle of Covid-19 and autoimmunity will not be easy. But if the pandemic has taught us anything, it’s that the virus won’t stand still. That means neither can we.
—
Adam Cohen is OMRF’s senior vice president and general counsel.
Read more from the Summer/Fall 2022 issue of Findings
Taking Pride
Voices: Dr. Padmaja Mehta-D’souza
Mouse Doctor
Ask Dr. McEver: Coffee Conundrum
Good Chemistry
Legacy of Giving
Predicting MS Relapses
Changing the Complexion of Science
Starring Role



