Each week, OMRF Vice President of Research Dr. Rod McEver opens “Adam’s Journal” to answer a medical question from Adam Cohen, OMRF’s senior vice president & general counsel and interim president.
Adam’s Journal
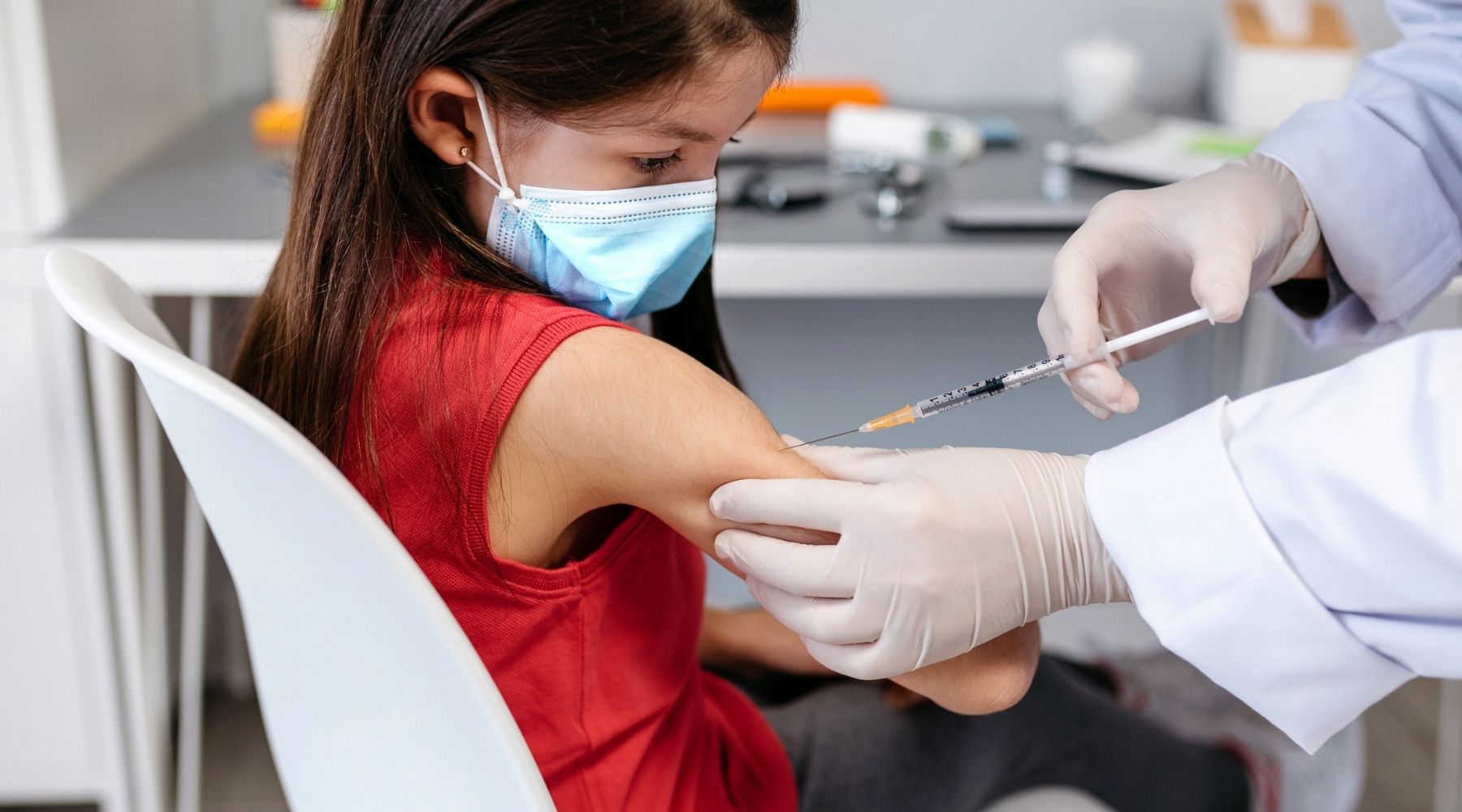
The Food and Drug Administration has approved Covid-19 vaccines for adults and for children and teens 12 and up. What’s taking so long for younger kids?
Dr. McEver Prescribes
Each of the three pharmaceutical companies with a Covid-19 vaccine approved for use in the U.S. wants to make its shot available to children under 12. Although there is no official timeline, FDA officials said last month they hope to have pediatric Covid-19 vaccines available “in the coming months.”
Pfizer is furthest along in testing their vaccine in clinical trials. The vaccine is the same chemical formulation, but the company is assessing different dosages for different age groups.
Last week, Pfizer submitted data to the FDA for children ages 5 to 11 who received its vaccine in a clinical trial. The drugmaker used a dosage of 10 micrograms per shot, one-third of what’s being administered to older children and adults. The company said those doses evoked similar antibody responses to those aged 16 to 25 who received 30 microgram doses.
Pfizer plans to submit a formal request for emergency-use authorization to the FDA for 5-to-11-year-olds in the coming weeks. The FDA expects to analyze that trial data in a matter of weeks, with review by the Centers for Disease Control and Prevention to follow soon after.
If both agencies offer green lights, elementary school-age children around the country may have access to Pfizer’s vaccine by late fall. Subsequent approvals for other age groups and manufacturers will follow the same process.
Although we know the coronavirus vaccines are safe and effective in adults, these thorough regulatory review processes exist because children can react differently to medications than adults. The processes may seem cumbersome, but they protect our children.
–
Do you have a health query for Dr. McEver? Email contact@omrf.org and your question may be answered in a future column!