In most households, Christmas came on Dec. 25. For me, it was 11 days later.
In most households, Christmas came on Dec. 25. For me, it was 11 days later.
That was when I received the letter from the site where I’d participated in the clinical trials of the Pfizer BioNTech Covid-19 vaccine.
In September, I’d been injected with two doses of something that was either the experimental vaccine or a squirt of saline solution. Despite clues – a sore arm, a positive antibody test – that I’d received the actual vaccine, I couldn’t know for sure, because the trial was blinded.
Since that time, and especially since the companies announced that their vaccine was 95% effective at preventing Covid-19 infections, I’d been eagerly scouring the news. In particular, I was sniffing for clues about whether the companies might unblind the trial so that study participants like me could know whether we’d gotten the real McCoy – and receive the vaccine if it turned out we’d only been injected with placebo.
Despite statements from the companies that they wanted to do just that, some scientists lobbied against it. In The New England Journal of Medicine, more than a dozen vaccine experts argued that once a placebo group disappears from a clinical trial, the chance to collect rigorous, long-term data about a coronavirus vaccine will also vanish.
But that desire for data had to be weighed against the health of the tens of thousands of people who’d volunteered for the trials. “You put yourself out there with that risk,” one participant told The New York Times. “I am owed that vaccine.”
Personally, I didn’t feel the companies “owed” me the vaccine. When I signed up for the trial, the study coordinator explained they’d be tracking me for two years to see how I fared. If the vaccine got approved during that time, I asked, would I find out whether I received it?

“That usually happens in trials like this,” she said. “But at this point, we just don’t know.”
Nevertheless, she explained, in that case, I could drop out of the trial and get vaccinated. When I asked Dr. Judith James, an immunologist and vice president of clinical affairs at the Oklahoma Medical Research Foundation, if getting vaccinated under those circumstances would pose any risk to me, she said it wouldn’t.
So that was my plan. Sort of.
You see, I did feel a pull to continue the trial regardless. In my 18 years at OMRF, I’d come to understand the value of clinical trials in assessing the safety and efficacy of experimental vaccines and treatments. Sure, I’d provided the sponsors with four months’ worth of weekly health reports, along with quite a few vials of blood and nasopharyngeal swabs. But what about the next 20 months? Researchers needed to track folks like me so they could compare the long-term safety and efficacy outcomes for those who’d received the vaccine to those who hadn’t.
Plus, I’m a runner. I like to finish what I start. (I’m still haunted by a college race I dropped out of more than 30 years ago.)
Against this backdrop, the letter I’d received a few days into the new year seemed like a godsend. Here was my chance to stay the course and get my results.
I scanned the letter, my eyes quickly drawn to the following passage: “The Vaccine Transition Option enables you to learn whether you received the investigational vaccine or placebo and, if you received the placebo, to have the option to receive the investigational vaccine within the trial.”
So that had been my plan. Sort of.
I contacted the study coordinator and told her I’d like to find out whether I’d received placebo or vaccine. “We’re required to go by the State phase system in the unblinding process for the study,” she replied. “Do you know what phase you would fall in?”
Hmm. I did a little research. I’m 52 years old and in good health. Although I’ve registered a few borderline high blood pressure readings in doctor’s offices over the years, I’ve never been diagnosed with hypertension or anything else that would qualify as a comorbidity for severe Covid-19. Still, as an attorney, for reasons that escape me, I do meet Oklahoma’s definition of “essential” personnel.
That makes me phase 3. I let the study coordinator know.
“We should be in phase 3 in a couple of weeks,” she said. “We’ll start calling and scheduling once we get through all the subjects in phase 2.”
In other words, sit tight.
Afterward, I questioned my decision. I mean, would it really have been so bad to fudge my answer a bit? I could easily have told her phase 2, and I felt 99% certain she would have taken whatever response I gave her. Plus, with those blood pressure readings, it wouldn’t technically have been a lie.
This is a conundrum like ones faced by so many right now. If I’ve found a way to jump the line and get vaccinated – even if it’s not, in the strictest sense, my turn – should I take it?
That, of course, is a question each of us must answer for ourselves. There are no Covid-19 ethics referees out there throwing yellow flags.
But maybe, just maybe, I got a signal from the universe later that day to play on. It happened when I visited OMRF’s Rheumatology Center of Excellence, where I’m participating in a Covid-19 antibody study. (I can’t get enough of guinea-pigging for research.)
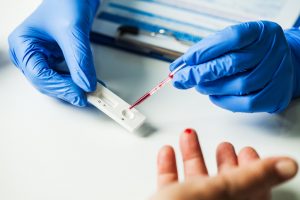
After the nurse took eight tubes of blood from my arm, she let me watch as she daubed a drop on one window of a lateral flow assay. Then, we sat and waited to see if a line appeared in another window of the test.
“It’s like testing for pregnancy,” she said. “It doesn’t matter whether it’s a faint line or a strong one. If it appears, you have antibodies.”
I stared at the tiny white box. I blinked my eyes over and over, hoping to make a line materialize. And soon enough, it did.
__
Read the rest of this series
Becoming a coronavirus vaccine guinea pig
My life as a vaccine guinea pig, part II
You’re here: My life as a vaccine guinea pig, part III
My life as a vaccine guinea pig, continued
Adam Cohen is OMRF’s senior vice president and general counsel. He can be reached at contact@omrf.org. Get On Your Health delivered to your inbox each Sunday — sign up here.