Rubber Chicken Bone
Rubber Chicken Bone
Materials:
- Chicken Bone, left over from a meal
- Lidded Jar, large enough for chicken bone and additional space
- Vinegar
Directions:
- Thoroughly clean all the meat from the bone.
- Rinse the bone under running water.
- Put the bone in the jar and cover it with vinegar.
- Seal the jar lid.
- Allow the jar to sit undisturbed for three days.
- Carefully remove the bone and rinse it under running water.
- Examine the bone. How does it feel?
How it Works:
The Vinegar is a mild acid and given enough time, the vinegar dissolves the calcium (which is a base) in the chicken bone.
Calcium doesn’t just build bones, it keeps the bone rigid.
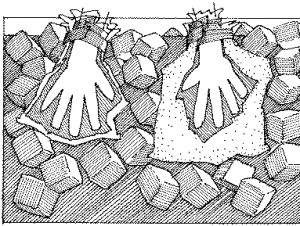
 Frankenstein’s Hand Experiment
Frankenstein’s Hand Experiment
Materials:
- 3 Tablespoons Vinegar
- 2 teaspoons baking soda
- Drinking glass
- Rubber glove
Directions:
- Pour the vinegar into the glass.
- Pour the baking soda into the inside of the glove. Hold the glove closed at the wrist and shake the powder into the fingers.
- Carefully attach the glove to the top of the glass. This needs to be an air tight seal, make sure there is no gap.
- Pull the glove upright by the fingertips and shake gently, allowing the baking soda to drop into the glass.
- Stand back and watch as the “hand” begins to come alive!
How it Works:
Baking soda is a chemical base which reacts strongly with the acid of the vinegar. The by-product of this chemical reaction between vinegar and baking soda is carbon dioxide. The carbon dioxide gas increases the pressure inside the glove-glass structure. As more carbon dioxide is produced, the pressure increases further and the gas gently inflates the rubber glove.
Do It Yourself Blubber
Materials:
1 cup vegetable oil
4 ziplock sandwich bags
Masking tape
Sink or deep bucket
Cold water and ice cubes
Timer
Directions:
- Add the vegetable oil to an open sandwich bag
- Turn a second sandwich bag inside out and insert it in the bag with the vegetable oil.
- Using the masking tape, seal any gaps where the bags join. This is your “blubber mitten.”
- Connect the other two sandwich bags together in the same way, only without any oil. This is your “normal mitten.” (each pair of bags becomes a “mitten.”)
- Fill the sink or bucket with cold water and add some ice cubes to lower the temperature.
- Put a bag mitten on each hand, start the timer, and submerge the mittens in the cold water.
- Time how long you can keep each bag mitten – blubber and normal, in the water before you have to pull it out because your hand is too cold.
How it Works:
The key to survival in very cold temperatures is preserving body heat. Blubber, the thick layer of fat under the skin of whales and seals, does that by insulating the body. In effect, it blocks out the flow of heat from the body to the outside. In this experiment, the oil insulates your hand the same way that blubber protects Arctic animals.
Petroleum Jelly could also be used as an insulator. What other household items could be insulators as well?