What did you say thanks for Thursday when you sat down for your turkey dinner? For Norman’s Mike Schuster, the blessings this year were in abundance when he gathered Thanksgiving at his in-laws’ home. “I have a lot to choose from,” said Schuster, 55.
What did you say thanks for Thursday when you sat down for your turkey dinner? For Norman’s Mike Schuster, the blessings this year were in abundance when he gathered Thanksgiving at his in-laws’ home. “I have a lot to choose from,” said Schuster, 55.
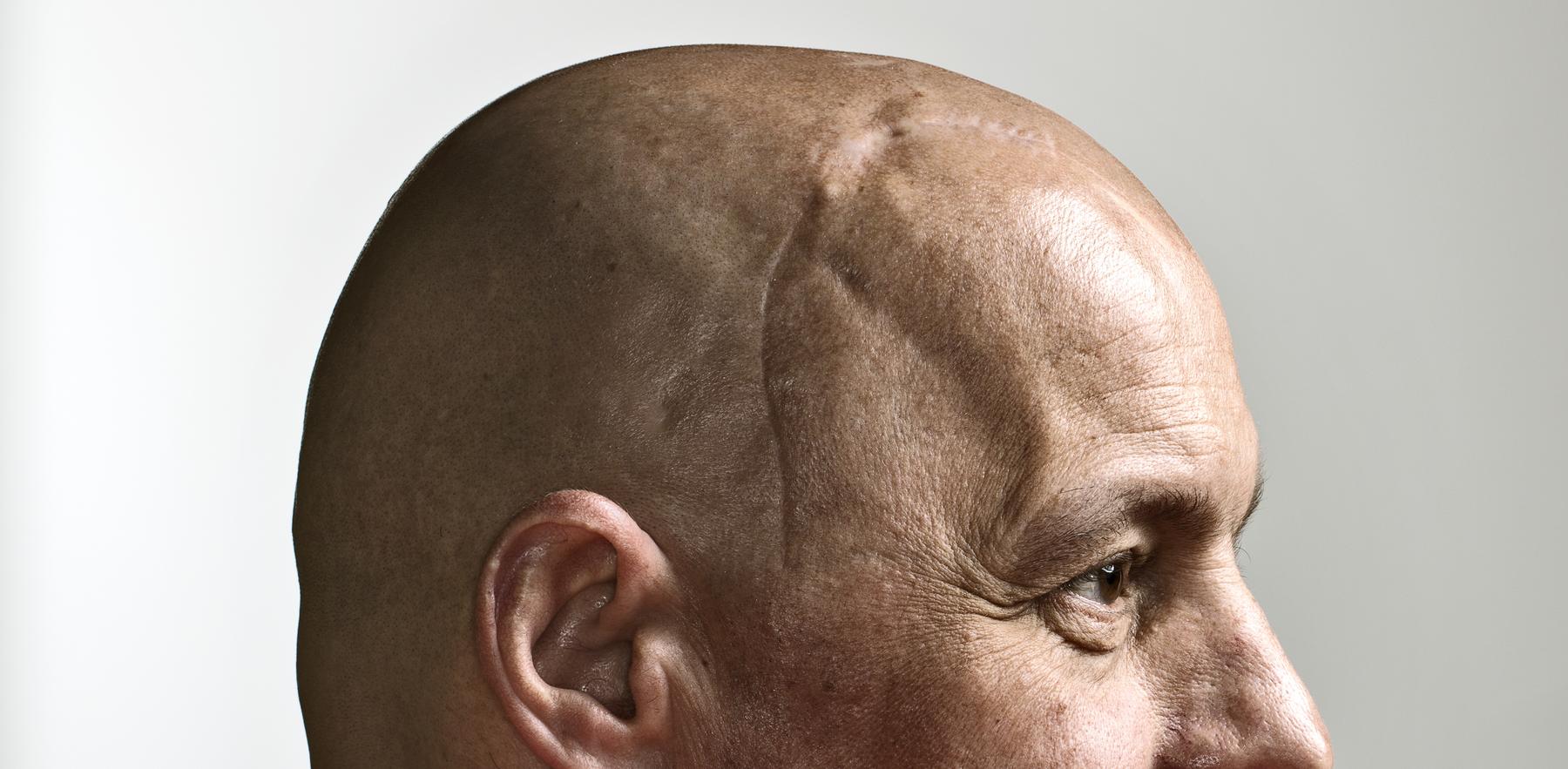
 On the day before Thanksgiving in 2015, Schuster suffered a seizure. At the hospital, doctors diagnosed him with glioblastoma, a deadly and aggressive form of brain cancer.
On the day before Thanksgiving in 2015, Schuster suffered a seizure. At the hospital, doctors diagnosed him with glioblastoma, a deadly and aggressive form of brain cancer.
That same cancer had taken the life of Beau Biden and Sen. Ted Kennedy, and it would soon claim Sen. John McCain. From diagnosis, the average life expectancy is less than 18 months. Only one in 20 patients survives five years.
Schuster, though, was determined to do all he could to beat those odds.
He underwent surgery to remove as much of the tumor as possible, then followed up with radiation and chemotherapy. Still, the tumor grew back, and 14 months after his initial surgery, Schuster underwent a second procedure.
Surgeons at the OU Health Stephenson Cancer Center were able to excise the primary tumor, but many of its tentacles escaped their reach, remaining lodged in his brain. “I’d thought that I had it beat,” Schuster recalled. “And now, I felt like I was starting all over again.”
Schuster knew those tentacles were, in essence, seeds that could sprout another full-blown tumor in a matter of weeks. He was willing to try anything to stop back from happening. He’d read about clinical trials of experimental medications, so he asked his doctor if there was one that might help him.
Schuster’s physician, Dr. James Battiste, was leading a trial that, he said, “just seemed suited for Mike.” The trial was designed to test an investigational I.V. treatment for glioblastoma.
 Coincidentally, that therapy had been born just down the block from Stephenson, at the Oklahoma Medical Research Foundation.
Coincidentally, that therapy had been born just down the block from Stephenson, at the Oklahoma Medical Research Foundation.
The experimental medication, known as OKN-007, was discovered by OMRF’s Drs. Rheal Towner and Robert Floyd. Schuster began treatment with it in early 2017.
Two years later, doctors declared Schuster cancer-free. And on Wednesday, he celebrated an anniversary he’d only dreamed possible: five years since his diagnosis.
Schuster attributes his against-the-odds survival to OMRF’s discovery. “That drug saved my life,” he said. Plus, “it was developed right here where I live. Where I raised my family.”
 That family consists of Teresa, his wife of 28 years, and two children, Connor and Parker. Since his diagnosis, Schuster has watched both sons graduate from college, and this past summer, he witnessed the addition of the newest family member, Julia, who married Parker in August.
That family consists of Teresa, his wife of 28 years, and two children, Connor and Parker. Since his diagnosis, Schuster has watched both sons graduate from college, and this past summer, he witnessed the addition of the newest family member, Julia, who married Parker in August.
“I’ve seen a lot I wasn’t expected to – moments I wouldn’t have wanted to miss for anything,” said Schuster. “God put me in the right place at the right time.”
In August, the Food and Drug Administration awarded OKN-007 a pair of designations that will provide special status and priority review applications for the medication. Before it reaches the FDA, though, the drug must continue to undergo clinical trials, a process we’ve all learned much more about as with the development of experimental vaccines and treatments for Covid-19.
For OKN-007, important parts of that process still remain. But based on preliminary results, OMRF’s Towner is optimistic.
“It’s going to be a drug that can provide real hope for patients,” he said. “I anticipate we’ll see a lot more stories like Mike’s in the future.”
__
Get Dr. Prescott’s column delivered to your inbox each Sunday — sign up here.